UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of registrant as specified in its charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s telephone number, including area code: |
|
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events
On May 9, 2023, Evoke Pharma, Inc. (“Evoke” or the “Company”), and EVERSANA, the Company's commercial partner, announced the details of the Company's presentation at Digestive Disease Week 2023 demonstrating that GIMOTI reduces healthcare resource utilization for patients with diabetic gastroparesis versus patients on oral metoclopramide.
Dr. David C. Kunkel, Gastroenterologist and Associate Professor of Medicine at UC San Diego Health, delivered the data to the AGA Distinguished Plenary Session. The study, guided by Dr. Kunkel and supported by EVERSANA’s Real World Evidence team, confirmed a hypothesis that improved symptom control in diabetic gastroparesis ("DGP") patients treated with nasal metoclopramide would result in lower healthcare resource utilization ("HCRU") compared to patients on oral metoclopramide. HCRU is the description and quantification of patients’ total usage of healthcare services such as hospitalization or how often they visit their physician in office.
Diabetic gastroparesis is an intermittent chronic disorder of the stomach characterized by delayed gastric emptying and a range of symptoms, including nausea, vomiting, early satiety, bloating, and abdominal pain, which drastically reduce a patient’s quality of life and can impair absorption of oral medications. According to a 2020 report, DGP patients experience three times greater emergency department ("ED") costs, three times greater inpatient admission costs, and two times greater outpatient costs compared to non-gastroparesis patients.
Given this debilitating condition places significant burden on patients and insurance payers, the researchers who conducted the study sought to compare the frequency of physician office, outpatient facility, ED, and inpatient hospital visits for patients with DGP treated with nasal metoclopramide ("NMCP") versus oral metoclopramide ("OMCP"). Select data points and key findings from the real-world data analysis are outlined below:
Image 1:
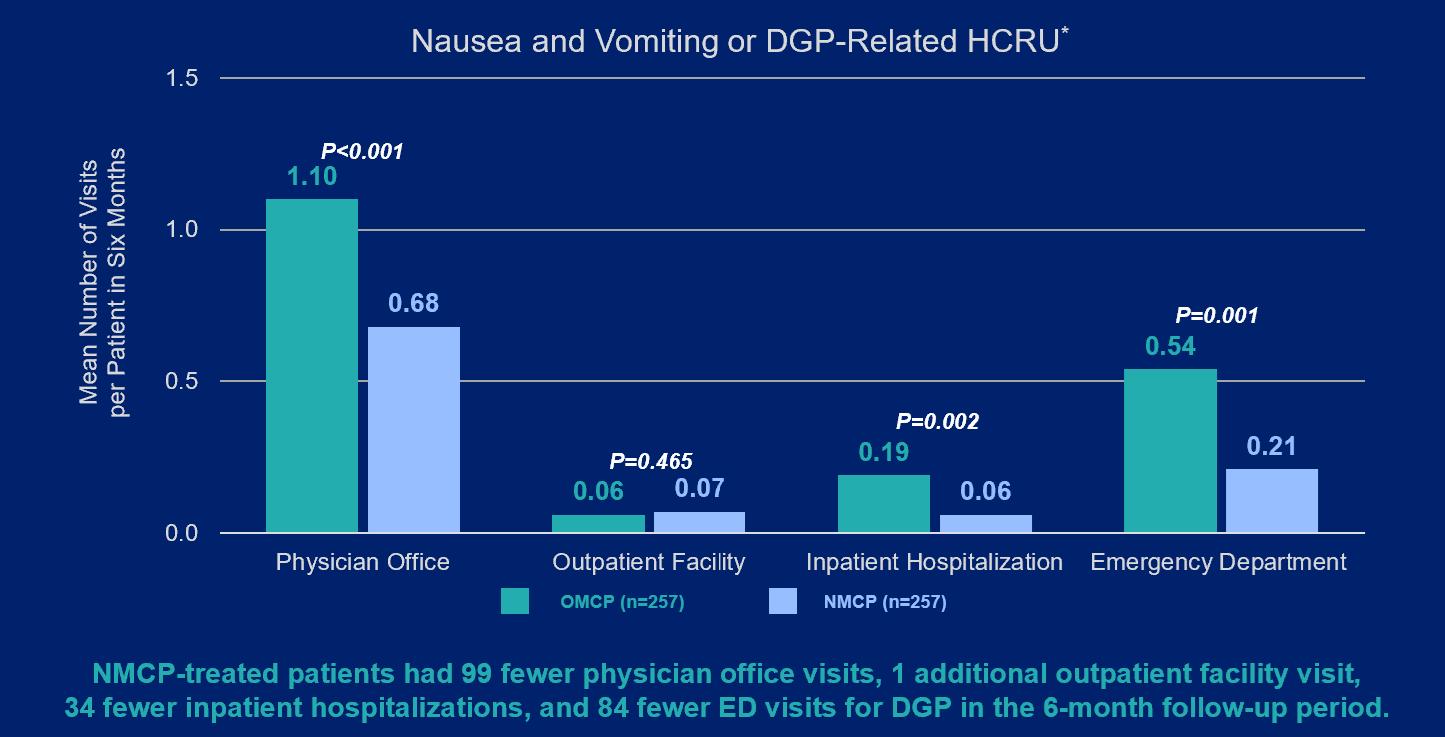
Image 2:
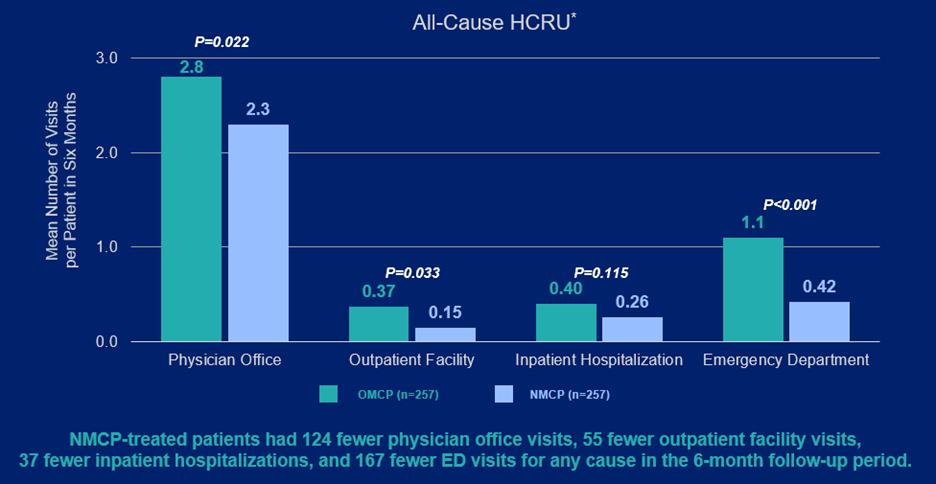
Image 3:

Conclusions:
Safe Harbor Statement
Evoke cautions you that statements included in this report that are not a description of historical facts are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negatives of these terms or other similar expressions. These statements are based on the Company’s current beliefs and expectations. These forward-looking statements include statements regarding: GIMOTI’s potential to reduce HCRU by diabetic gastroparesis patents; and Evoke’s belief that GIMOTI can improve treatment of diabetic gastroparesis. The inclusion of forward-looking statements should not be regarded as a representation by Evoke that any of its plans will be achieved. Actual results may differ from those set forth in this report due to the risks and uncertainties inherent in Evoke’s business, including, without limitation: the results of market research studies may not predict acceptance by patients, healthcare providers or payors; Evoke’s ability to obtain, maintain and successfully enforce intellectual property protection for GIMOTI; Evoke’s and EVERSANA’s ability to successfully drive market demand for GIMOTI; Evoke’s ability to obtain additional financing as needed to support its operations; the COVID-19 pandemic may continue to disrupt Evoke’s and EVERSANA’s business operations impairing the ability to commercialize GIMOTI and Evoke’s ability to generate any product revenue; Evoke’s dependence on third parties for the manufacture of GIMOTI; Evoke is entirely dependent on the success of GIMOTI; inadequate efficacy or unexpected adverse side effects relating to GIMOTI that could result in recalls or product liability claims; and other risks and uncertainties detailed in Evoke’s periodic reports it files with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Evoke undertakes no obligation to revise or update this report to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
EVOKE PHARMA, INC. |
|
|
|
|
Date: |
May 9, 2023 |
By: |
/s/ Matthew J. D'Onofrio |
|
|
|
Name: Matthew J. D'Onofrio |
