Press Release
Evoke Pharma Reports Fourth Quarter and Full Year 2023 Financial Results
Fiscal year 2023 net product sales from prescriptions totaled approximately
Company projects
"In 2023, we focused on operational excellence to expand GIMOTI’s market share and ensure its availability for patients requiring an improved gastroparesis treatment. Our commercial team’s dedicated efforts yielded a 107% increase in year-over-year revenue,” stated
Fourth Quarter and Full Year 2023 Developments and Recent Highlights:
- Continued Advocacy for GIMOTI as the Standard of Care for Diabetic Gastroparesis Treatment
- Healthcare resource utilization data presented at DDW 2023 revealed that there is statistically signficantly less burden on healthcare resources and facilities such as hospitical admissions, emergency department and physician office visits with patients who are being prescirbed GIMOTI versus those on oral metoclopramide.
- Based on the reduced utilization data, additional compelling cost data presented in a distinguished plenary session at ACG 2023 demonstrated financial savings benefits of GIMOTI for patients and payors compared to oral metoclopramide.
- Abstract focused on the HCRU of diabetic gastroparesis care in women using nasal metoclopramide to be presented at DDW 2024.
Transitioned Pharmacy Service Partnership to ASPN Pharmacies- Aiming to enhance patient prescription process and enhance revenues through ASPN’s extensive network of partners with broader PBM agreements.
Fortified Patent Estate for GIMOTI- Two patents issued in 2023 covering the methods of use for GIMOTI including two Orange Book listings.
Teva Pharmaceuticals determination not to pursue a paragraph 4 certification against Gimoti without financial or other consideration further enhanced Evoke’s intellectual property position and eliminated 180-day exclusivity opportunity for later possible generic seeking entities for the future.
- Improved Cash Position
- In
February 2024 , the Company closed a$7.5M public offering with fundamental, healthcare-oriented institutional investors providing the company runway into the fourth quarter of 2024 with up to an additional$22.5M available if common stock warrants are exercised in full.
- In
Fourth Quarter and Full Year 2023 Financial Review and Outlook
For the fourth quarter of 2023, net product sales were approximately
- Prescription sales through pharmacy service partnership with
ASPN Pharmacy ; - Recapture of prescriptions sent to retail pharmacies without ability to order product; and
- Marketing of head-to-head real-world data comparing Gimoti to oral metoclopramide showing improvements in fewer hospitalizations and ER visits with GIMOTI.
Research and development expenses totaled approximately
For the fourth quarter of 2023 selling, general and administrative (SG&A) expenses were approximately
Total operating expenses for the fourth quarter of 2023 were approximately
As of
Evoke projects net revenue in 2024 of approximately
About Evoke Pharma, Inc.
Evoke is a specialty pharmaceutical company focused primarily on the development of drugs to treat GI disorders and diseases. The company developed, commercialized and markets GIMOTI, a nasal spray formulation of metoclopramide, for the relief of symptoms associated with acute and recurrent diabetic gastroparesis in adults.
Diabetic gastroparesis is a GI disorder affecting millions of patients worldwide, in which the stomach takes too long to empty its contents resulting in serious GI symptoms as well as other systemic complications. The gastric delay caused by gastroparesis can compromise absorption of orally administered medications. Prior to FDA approval to commercially market GIMOTI, metoclopramide was only available in oral and injectable formulations and remains the only drug currently approved in
Visit www.EvokePharma.com for more information.
Follow GIMOTI on Facebook
Follow Evoke Pharma on Facebook
Follow Evoke Pharma on LinkedIn
Follow Evoke Pharma on Twitter
About Gimoti® (metoclopramide) nasal spray
GIMOTI is indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. Important Safety Information
WARNING: TARDIVE DYSKINESIA
Metoclopramide can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. The risk of developing TD increases with duration of treatment and total cumulative dosage.
Discontinue GIMOTI in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after metoclopramide is stopped.
Avoid treatment with metoclopramide (all dosage forms and routes of administration) for longer than 12 weeks because of the increased risk of developing TD with longer-term use.
GIMOTI is not recommended for use in:
Pediatric patients due to the risk of developing tardive dyskinesia (TD) and other extrapyramidal symptoms as well as the risk of methemoglobinemia in neonates.
Moderate or severe hepatic impairment (Child-Pugh B or C), moderate or severe renal impairment (creatinine clearance less than 60 mL/minute), and patients concurrently using strong CYP2D6 inhibitors due to the risk of increased drug exposure and adverse reactions.
GIMOTI is contraindicated:
In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide.
When stimulation of gastrointestinal motility might be dangerous (e.g., in the presence of gastrointestinal hemorrhage mechanical obstruction, or perforation).
In patients with pheochromocytoma or other catecholamine-releasing paragangliomas. Metoclopramide may cause a hypertensive/pheochromocytoma crisis, probably due to release of catecholamines from the tumor.
In patients with epilepsy. Metoclopramide may increase the frequency and severity of seizures.
In patients with hypersensitivity to metoclopramide. Reactions have included laryngeal and glossal angioedema and bronchospasm.
Potential adverse reactions associated with metoclopramide include: Tardive dyskinesia (TD), other extrapyramidal effects (EPS), parkinsonism symptoms, motor restlessness, neuroleptic malignant syndrome (NMS), depression, suicidal ideation and suicide, hypertension, fluid retention, hyperprolactinemia, effects on the ability to drive and operate machinery. Most common adverse reactions (≥5%) for GIMOTI are: dysgeusia, headache, and fatigue. These are not all of the possible side effects of GIMOTI. Call your doctor for medical advice about whether you should take GIMOTI and the possible risk factors and side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Safe Harbor Statement
Evoke cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negatives of these terms or other similar expressions. These statements are based on the company’s current beliefs and expectations. These forward-looking statements include statements regarding: guidance regarding 2024 net product sales; potential future prescribing trends for GIMOTI based on Evoke’s or EVERSANA’s marketing efforts; Evoke’s commercialization plans, including the potential that GIMOTI could become the standard of care for gastroparesis; the potential for additional funds from the exercise of outstanding warrants and Evoke’s expected cash runway. The inclusion of forward-looking statements should not be regarded as a representation by Evoke that any of its plans will be achieved. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in Evoke’s business, including, without limitation: Evoke may not be able to achieve it’s guidance for 2024 including as a result of decreased demand for GIMOTI; Evoke’s and EVERSANA’s ability to successfully drive market demand for GIMOTI; Evoke’s ability to obtain additional financing as needed to support its operations; Evoke may use its capital resources sooner than expected; warrant holders may choose not to exercise any of the outstanding warrants; Evoke’s dependence on third parties for the manufacture of GIMOTI; Evoke is entirely dependent on the success of GIMOTI; inadequate efficacy or unexpected adverse side effects relating to GIMOTI that could result in recalls or product liability claims; Evoke’s ability to maintain intellectual property protection for GIMOTI; and other risks and uncertainties detailed in Evoke’s prior press releases and in the periodic reports it files with the
Investor & Media Contact:
Tel: 862-213-1398
dboateng@dkbpartners.net
Financial Statements to Follow
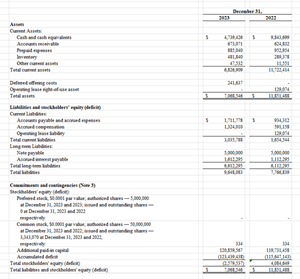
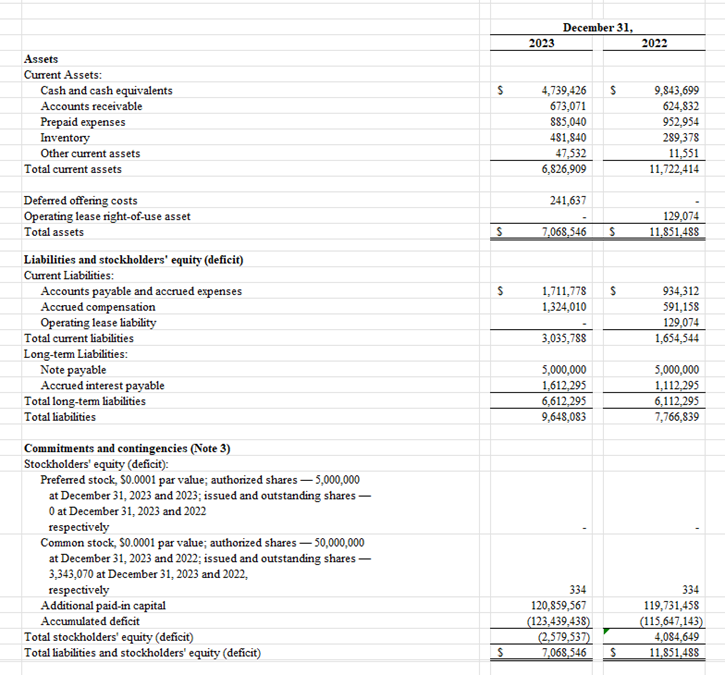
Balance Sheet
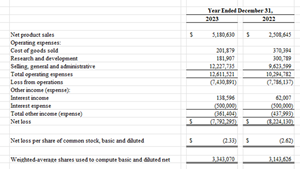
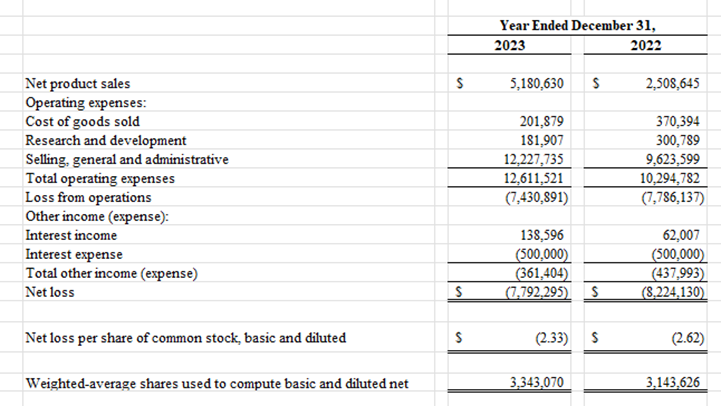
Statement of Operations
The Financial Statements accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/1505239a-5694-4a7e-99c2-8d96e50a84a3
https://www.globenewswire.com/NewsRoom/AttachmentNg/b26e2618-665c-4966-acc6-c45d683cb7e0

Source: Evoke Pharma, Inc.